TROG Cancer Research received international recognition last year at the October American Society for Radiation Oncology (ASTRO) Annual Meeting on the research and findings of the TROG 13.01 SAFRON II trial.
Chief Investigator of the trial, Associate Professor Shankar Siva presented outcomes of the SAFRON II trial to leading radiation oncology professionals around the world and discussed how stereotactic body radiation therapy (SBRT) could be used to reduce the number of treatment sessions required and provide an alternative to common drug therapies.
Conducted across 13 radiation oncology centres in Australia and New Zealand, the SAFRON II trial strengthens the case for radiation therapy as a treatment for cancer that has started to spread to further regions in the body.
What the trial uncovered was that patients who had up to three lung metastases were able to benefit equally from SBRT treatment, despite whether it was delivered in one or four sessions.
A/Prof. Siva said it is trials like this that help develop treatment options that are more effective and less invasive than existing methods.
“Our results indicate that SBRT may be a safe and effective treatment for patients whose cancer has spread to the lungs, even when administered in a single session,” he said.
“When we compress a cycle of multiple treatments into one treatment, there is a potential risk of increased toxicity.
“In this study, just a year later, we are seeing similar efficacy, where 93-95 per cent of tumors were controlled in both arms. Our final analysis will show whether this holds in the long term, but these early results indicate that single fraction radiation could be carried out just as effectively in multiple institutions.”
For cancer patients, this is a breakthrough in ensuring quality of life remains at the forefront of cancer treatment, which has always been the number one goal for TROG Cancer Research.
CEO of TROG Cancer Research, Susan Goode said that up to half of all cancers (excluding primary lung cancer) tend to spread to the lungs.
The findings of the SAFRON II trial give cancer patients of today and in the future a chance to enjoy more time with family and friends, and less time in hospital receiving treatment.
“Cancer tumours are stubborn. To be able to present a treatment option that allows for patients to live without the fear of cancer returning is a gift we are always working towards,” Susan said.
“For patients with a limited number of metastases, recent studies have shown that there may be long-term survivors with the use of SBRT,” A/Prof Siva said.
“These studies tend to be smaller institutional series with a wide variety of SBRT regimens, so we designed our trial to test the safety and efficacy of SBRT in a more robust way.”
In this Phase II TROG Cancer Research trial, A/Prof Siva and his team randomly placed 90 patients into two treatment arms: half receiving a single fraction of 28 Gy and the other half receiving a biologically equivalent regimen of four fractions of 12 Gy each.
Each patient had up to three lung metastases from primary tumors from other body sites, most commonly colorectal cancer (47 per cent).
A total of 37 patients in each treatment group were eligible for safety analyses one year after treatment. In the single treatment cohort, two patients had grade three side effects, including fatigue, shortness of breath, and chest pain. However, there were no patients who experienced grade four or five side effects such as hospitalisation or death.
In addition to the analyses, the research compared survival rates between the groups a year after treatment and found the results to be nearly identical across both regimens. A/Prof Siva said this will be something their team will continue to monitor to ensure optimal effectiveness.
With the COVID-19 pandemic there has been an encouragement of these shorter-cycle therapies, as medical institutions seek ways to reduce potential exposure, especially among vulnerable cancer patients.
“In a pandemic, the idea of a single non-invasive outpatient treatment that does not require anesthesia is attractive in the sense of reducing patient time and risk of transmission in the clinic,” A/Prof Siva said.
Susan said it is incredible to be able to share these innovative solutions with leading health professionals internationally and present cancer patients with hope for the future.
“Our team are consistently aiming to find treatment options that put the needs of our cancer patients first. So, to get the opportunity to present this innovative research and further solidify our position as a leader in global cancer treatment is an honour,” Susan said.
“A/Prof Siva and the SAFRON II trial team should be very proud of their hard work and this achievement.”
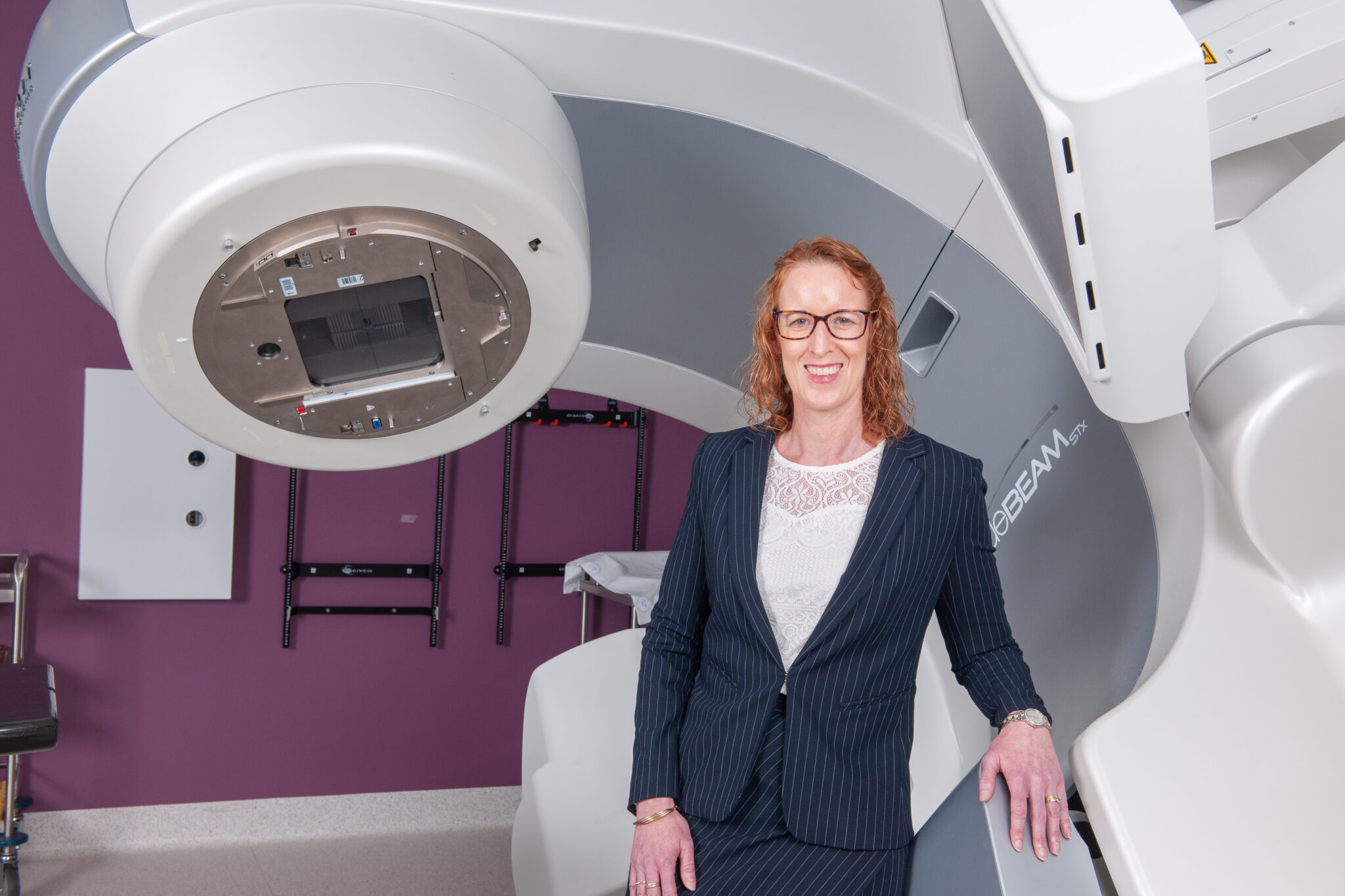
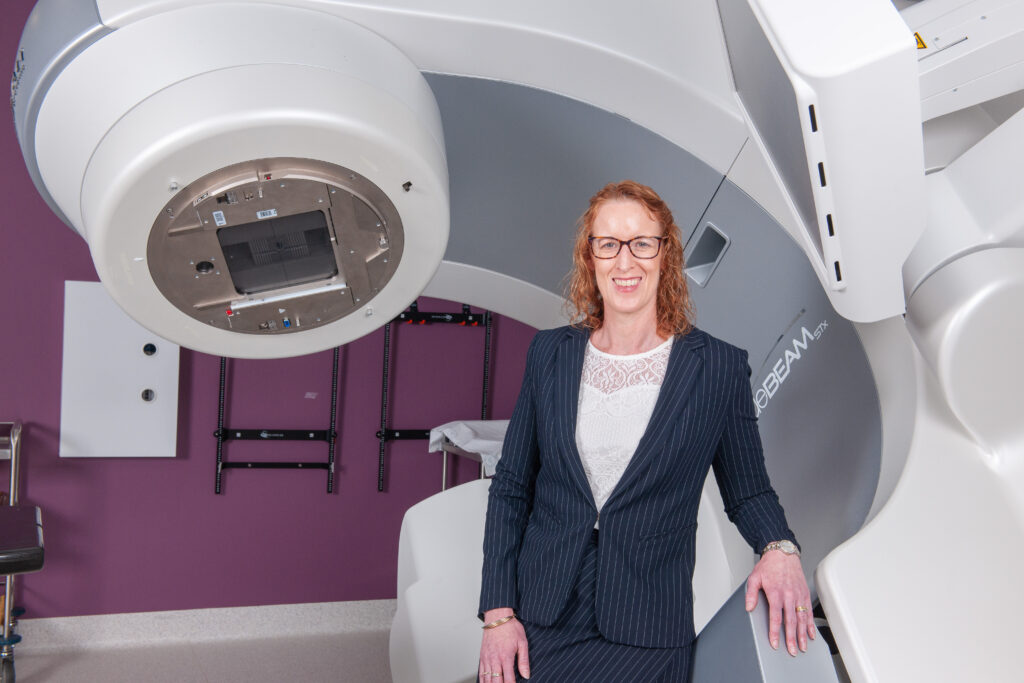
IMAGE | TROG Cancer Research CEO, Susan Goode, and radiation therapy machine.